| This Month On IVT Network |
| How to Identify a GMP Project |

|
A GMP product is a project wherein there is an effect on equipment, control system, software, process, or facility (ECS-SPF) that has a direct impact those quality characteristics. Read More |
| Process Validation: Begin with the End in Mind—An Industry Survey on Continued Process Verification |
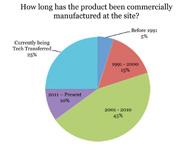 |
The Pharmaceutical Regulatory Science Team (PRST), a research team based at the Dublin Institute of Technology (DIT) in Ireland, conducted a recent industry survey. Read More |
| Implementing Risk into the 3 Stages of Process Validation |
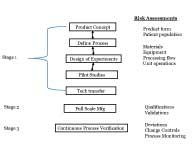 |
Process Design, Process Qualification, and Continued Process Verification; has their own set of risk assessments that are dependent on the activities required in that stage. Read More |
| IVT PRODUCTS WITH YOUR MEMBERSHIP |
| Continuing Process Verification |
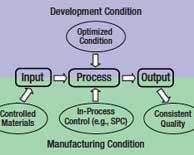 |
Covering best practices to ensure compliance and expert insight on monitoring and maintaining the validated state. Read More |
| Roadmap to GMP Compliance Part I |

|
Covering all the bases from GMP failures to reviewing batch production records and beyond. Read More |
| Conducting Audits, Gap Assessments & Corrective Actions |

|
Expert insight combined with generic SOPs, checklists, and master validation plans for manufacturers will ensure compliance for any medical device or pharmaceutical. Read More |
| Establishing a Valuable QbD Programme |
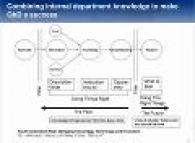 |
The current understanding and expectations for implementation of QbD are described including approaches, benefits, and potential pitfalls, especially for regulatory applications. Read More |
| LATEST JOURNAL ARTICLES |
| Statistical Analysis in Analytical Method Validation |
 |
The objective of this paper is to provide an overview of regulatory expectations related to statistical analysis and the review of common statistical techniques used to analyze analytical method validation data.
Read More |
| Validation of a Cleaning Process for Medical Devices |
 |
Many medical device manufacturers find it a considerable challenge to plan and conduct a cleaning validation. Read More
|
| Harmonized Microbial Limits Testing Validation Strategies |
 |
Key factors in developing a proper method include some experimentation as well as knowledge of the pH of the material, the water activity of the material, the water solubility of the material, and any antimicrobial properties of the material, to name a few. Read More
|
| Specifications for Transdermal Drugs |
 |
Developing specifications for transdermal drug products is very challenging in that it requires a multi-functional approach. Read More |
| Developing a Train-the-Trainer Program for Regulatory Compliance Part I |
 |
The first part focuses on the foundations of such a TTT program, addressing the processes involved in preparing for, conducting, and documenting the various sessions that make up the TTT program. Read More |
| |
|
|