Top Story

|
Valentina D’Atri from the University of Geneva spoke to The Column about the evolving role of HILIC in biopharmaceutical analysis. read more
|
advertisment
One of the newest and most exciting fields in analytical testing is medical and retail marijuana. With new legislation passing for the distribution of marijuana and marijuana related products, an urgent need for fast, reliable and economical testing protocols exists. Both the product purity and potency are of major concern from a quality control and patient consumer safety standpoint.
 Learn more Learn more
|
|
|
|
FEATURES

|
The rapid growth of biologics in development, the increasing demand for more robust analytical technologies to directly monitor the critical quality attributes (CQAs) of these new drugs, and longer term industry initiatives aimed at improving quality and productivity, such as quality by design (QbD) regulatory submissions and continuous manufacturing, are all fueling a greater need for mass monitoring with MS. read more
|

|
Biopharmaceutical purification techniques can be slow and cumbersome with poor scalability. A new device using laterally-fed membrane chromatography (LMFC) was developed to address these issues. Raja Ghosh from McMaster University in Hamilton, Ontario, Canada, spoke to The Column about this new device and its potential applications. read more
|
|
|
|
Application Notes
advertisment
Improved resolution and peak shape performance for the determination of blood alcohol concentration using J&W DB-BAC1 Ultra Inert and DB-BAC2 Ultra Inert columns.  Learn more Learn more
advertisment
The Agilent Intuvo 9000 gas chromatograph addresses issues associated with multiresidue pesticide analysis of food products.  Learn more Learn more
more FEATURES

|
The chromatographic methods used to determine drug-to-antibody ratio (DAR), including hydrophobic interaction chromatography (HIC), reversed phase, and liquid chromatography–mass spectrometry (LC–MS) are discussed. read more
|
NEWS

|
Researchers have developed and validated a stability- and potency-indicating assay protocol for high-throughput quality assessment of vaccines consisting of recombinant virus- "like" particles (VLPs) using conformation-dependent antibodies coupled to size-exclusion high performance liquid chromatography (SE-HPLC). read more
|
|
|
|
FEATURED PRODUCT
|
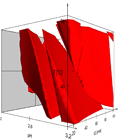
DryLab®4 is the (U) HPLC method development and optimization software that predicts chromatograms under a much wider range of experimental conditions than would ever be possible in the laboratory.
 / Learn more / / Learn more /
|
|
|
|
|
|