|
This Month On IVT Network |
| The FDA Perspective on CAPA |
 |
FDA's expectations for a company's corrective and preventive action (CAPA), as outlined in Code of Federal Regulations Title 21 Part 820. Read More |
| The Validation Quality System Lifecycle |
 |
How companies can apply the process validation lifecycle to their validation quality systems. Read More |
| Lifecycle Approach to Equipment Qualification |
 |
The lifecycle approach to process validation as it applies to equipment qualification. Read More |
| |
| IVT PRODUCTS WITH YOUR MEMBERSHIP |
| Cleaning Validation Volume III |
 |
In this installment of IVT's Conducting Audits Special Edition Series, both third-party, internal, and regulatory agency inspections are discussed. Read More |
| Validation Essentials |
 |
This webinar will discuss the fundamentals of effective and US Food and Drug Administration-compliant process validation. Read More |
| 14th Annual Computer and Software Validation |
 |
Computer and software validation practices are quickly evolving with the emergence of virtual networks, cloud computing and social media. Read More |
| LATEST JOURNAL ARTICLES |
| Use of a Two Tiered Degree-Hour Measure for the Management of Investigational Medicinal Product Over-Temperature Excursions Using Mean Kinetic Temperature (MKT) |
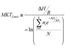 |
This manuscript translates the USP Pharmacopeial Mean Kinetic Temperature (MKT) into an approach that can be applied to temperature excursion management. Read More |
| API Pharmaceutical Water Systems Part II: Water System Validation |
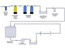 |
The validation of a pharmaceutical water system for the active pharmaceutical ingredient (API) industry will depend on the type of API process. Read More
|
| Variations in the Resistance of Biological Indicators Used to Assess Sterilization |
 |
Biological indicators (BI) are routinely used within the pharmaceutical, food, and medical devices industries to monitor the efficacy of various sterilization processes. Read More
|
| The GUDID |
 |
The UDI Final Rule is FDA’s first step towards requiring regulated device manufacturers to implement a consistent way to identify medical devices throughout their distribution and use. Read More |