|
This Month On IVT Network |
| A Risk Management Solution Designed to Facilitate Risk-Based Qualification, Validation, and Change Control Activities |
 |
In the European Union (EU), the Good Manufacturing Practice (GMP) requirements place specific obligations on manufacturers of medicinal products to implement risk-based qualification, validation, and change control programmes. Read More |
| Cleaning Validation Proposed Standard |
 |
The following proposed standard is intended to reflect desirable contemporary practices, is not binding in any way, and can be modified to suit a firm's specific needs. Read More |
| Design Process: Established Need to Final Form |
 |
The purpose of this article is to define a systematic design approach that can be used to streamline the design, purchase, and validation of systems used in the manufacture of life science products. Read More |
| |
| IVT PRODUCTS WITH YOUR MEMBERSHIP |
| Conducting Audits, Gap Assessments, & Corrective Actions Volume II |
 |
In this installment of IVT's Conducting Audits Special Edition Series, both third-party, internal, and regulatory agency inspections are discussed. Read More |
| HVAC Operation Qualification Protocol |
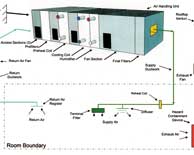 |
This protocol will be executed in compliance as per the requirements in 21CFR 210 & 211, ICH Q-7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients, August 2001. Read More |
| Equipment, Systems, and Processes Revalidation SOP |
 |
This procedure applies to previously validated facilities, equipment, systems, and processes that are used to manufacture or to support manufacturing. Read More |
| LATEST JOURNAL ARTICLES |
| Soil Spreading and Image Analysis Methods to Control and Quantify Soil Morphology and Establish Worst-Case Soils in Support of Risk-Based Cleaning Validation Strategies |
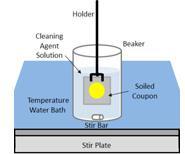 |
Bench-scale methods for assessing the relative cleanabilities of process soils are useful tools for applying a risk-based approach to full-scale cleaning validations, troubleshooting cleaning issues, and developing cleaning strategies. In this study, the authors explored a coupon soiling method, referred to as the “spreading method,” that controls soil size and shape during sample preparation. Read More |
| Electronic Batch Records: Best Practices for Implementation and Validation |
 |
Electronic batch record systems (EBR) are increasingly commonplace in pharmaceutical and biotechnology manufacturing environments. Read More
|
| Molding a Single-Use Plastic Part: From Design to Validation According to the FDA Process Validation Guidance |
 |
This paper discusses step-by-step how the recent evolution of process can be applied to new molded parts used in the regulated industry. Read More
|
| Translating Laboratory-Developed Visual Residue Limits to Process Area Applications |
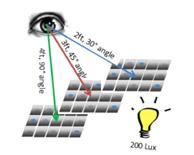 |
Many factors influence how visual inspection will be conducted in a manufacturing facility. Read More |
| Technical/Quality Agreements |
 |
Outsourcing activities are often of a complex nature and carry significant risks from a commercial and a compliance perspective. Read More |
| |
|