|
This Month On IVT Network |
| Webinar on March 11th: Process Monitoring & Metrics to Comply with FDASIA 705/706 |
 |
While it’s unlikely that 100 million defects are in the market, it is possible to have 100 million non-conformances in the market without widespread patient impactl. Read More |
| The Configuration Management Process |
 |
The configuration management activities for computerized systems were thoroughly detailed in this presentation from IVT's Change Control. Read More |
| Options for Financially Supporting GLP and GCLP Quality Assurance Programs within an Academic Institution |
 |
This discussion provides examples of how academic institutions can financially support QA functions for animal and laboratory quality-controlled studies. Read More |
| 3 Strategies for Surviving Change in the Laboratory |
 |
Change control in the analytical laboratory is a lengthy and multi-departmental process. Read More |
| IVT PRODUCTS WITH YOUR MEMBERSHIP |
| 21 CFR Part 11 Compliance: Facts & Myths |
 |
Complying to 21 CFR Part 11 during a period of constant digital innovation brings is discussed in this webinar. Read More |
| 18th Annual Validation Week |
 |
The collection of all presentations given IVT’s 18th Validation Week conference by industry experts and thought-leaders. Read More |
| CAPA and Design Control Warning Letter |
 |
A review of recent warning letters and recent FDA inspections will provide insight into what you need to know about complying with these important areas. Read More |
| LATEST JOURNAL ARTICLES |
| Validation Activities Related to the SaaS Application: Best Practices |
 |
In the past five years, cloud computing adoption has grown steadily. Key steps for validation best practices for a SaaS are illustrated. Read More |
| Change Control and the Validated System |
 |
A dilemma occurs when the change control process and the computer system validation process have to meet. Read More
|
| Proper Remediation Planning for FDA 483 Observations: A Firm's Best Approach in Avoiding a Warning Letter |
 |
Management will need to ensure a clear and scientific strategy is developed and communicated to FDA. Read More
|
| Quality and Validation: Can We Get Along Together? |
 |
Historically, the relationship between Quality and Validation has been dysfunctional, unproductive, and inefficient. These issues have had a negative impact in the operation of each organization. Read More |
| Process Validation: Begin with the End in Mind—An Industry Survey on Continued Process Verification |
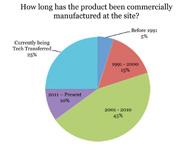 |
The Pharmaceutical Regulatory Science Team conducted a recent industry survey to assess the level of process verification implementation in the pharmaceutical industry. Read More |
| |
|