| This Month On IVT Network |
| 6 Considerations for QbD Use in Stability Studies |
 |
Quality-by-design (QbD) is a systematic approach whereby desired traits and performance are built into a process. Read More |
| Methodology for Assessing Product Inactivation During Cleaning Part II: Setting Acceptance Limits of Biopharmaceutical Product Carryover for Equipment Cleaning |
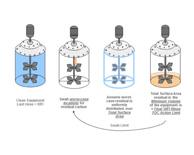 |
Alternative approaches for setting acceptable levels of process residue will be described building upon the basis that API inactivation by the cleaning process has been demonstrated. Read More |
| 6 Steps to Compliant Equipment Qualification |
 |
Equipment qualification will provide documented evidence that the subject equipment has been installed per specification and will attain and maintain critical process parameters repeatedly and reliably. Read More |
| IVT PRODUCTS WITH YOUR MEMBERSHIP |
| Method Validation Toolkit |
 |
This toolkit highlights the most comprehensive information on analytical method validation and method development. Read More |
| Installation Qualification Protocol |
 |
Based on the outcome from the DQ and initial equipment evaluation, such as testing of the equipment at the vendor site (e.g., FAT), and/or SAT conducted upon initial receipt of the equipment, a formal Installation Qualification (IQ) protocol can be developed. Read More |
| cGMP Computerized System Vendor Audit Questionnaire |
 |
While in the process of choosing and auditing a vendor, using this questionnaire will ensure that the best choice is made for your company. Read More |
| LATEST JOURNAL ARTICLES |
| Compendial Water Systems—Proactive Preventative Maintenance Part I |
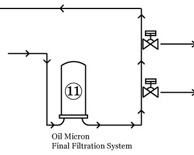 |
Over the past several years, harmonization of compendial water specifications has generated a debate regarding the method of producing Water for Injection (WFI) in bulk. Read More |
| CpK Distribution: The Fact Underlying Process Capability Indices—Part I: Theory |
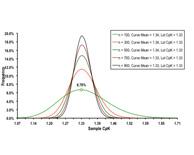 |
Process capability index (CpK) is one of the most commonly used statistical parameters for measuring and improving the processes as far as statistical process control (SPC) is involved. Read More
|
| Understanding, Preventing, and Remediating Mold in Cleanrooms |
 |
Mold contamination has been considered a hazard in residential and commercial buildings for many years. In the past few years, however, mold contamination issues in pharmaceutical products have caught the attention of regulators. Read More
|
| Quality-by-Design for Analytical Procedures |
 |
Quality-by-design (QbD) is being used successfully for production processes; the same QbD approach can be applied to analytical procedures. Read More |
| Developing a Train-the-Trainer Program for Regulatory Compliance Part I |
 |
The first part focuses on the foundations of such a TTT program, addressing the processes involved in preparing for, conducting, and documenting the various sessions that make up the TTT program. Read More |
| |
|