| This Month On IVT Network |
| 4 Fundamentals of Effective Validation Master Plans |

|
A VMP is a document that declares how a validation will satisfy the relevant regulatory requirements. Read More |
| Resolving the 3 Types of Validation Exceptions |
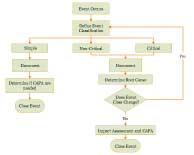 |
A validation exception is an error or failure that occurs during validation or verification. Read More |
| 6 Strategies to Survive an FDA Inspection |
 |
How you prepare for the inspection, handle the inspection, and the action you take to follow up the inspection can be the difference between a good inspection or one that results in regulatory correspondence. Read More |
| IVT PRODUCTS WITH YOUR MEMBERSHIP |
| 19th Annual Validation Week Compendium |
 |
Validation Week is the forefront of promoting good validation practice for the last three decades. Read More |
| Computer and Software Validation Volume II |
|
Computer and software validation practices are quickly evolving. The urgency of securing data integrity and complying with global regulations has never been greater. Read More |
| Analytical Method Validation Volume III |
|
Analytical Method Validation Volume III highlights application of method validation in the laboratory; implementation of process improvement strategies; relevance of global method validation; use of Monte Carlo Simulation; and validation of dissolution methods. Read More |
| Establishing a Valuable QbD Programme |
 |
The current understanding and expectations for implementation of QbD are described including approaches, benefits, and potential pitfalls, especially for regulatory applications. Read More |
| LATEST JOURNAL ARTICLES |
| Estimating the Shelf Life of a Single Batch |
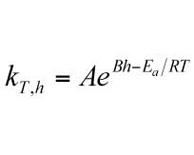 |
Current approaches to estimation of batch shelf life for pharmaceutical products are not satisfactory. An alternative approach, based upon measuring shelf life directly, can be used in conjunction with an expanded Arrhenius model to predict shelf life under unstressed conditions based upon shelf life measured under stressed conditions.
Read More |
| Process Performance Qualification: Selecting the Optimal Sampling Plan |
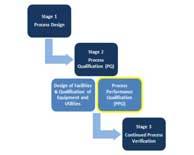 |
Process validation is defined as the collection and evaluation of data from the process design stage through the commercial production that establishes scientific evidence that a process is capable of consistently delivering quality product and thereby also assuring reliability of supply. Read More
|
| Specifications for Transdermal Drugs |
 |
Developing specifications for transdermal drug products is very challenging in that it requires a multi-functional approach. Read More |
| Developing a Train-the-Trainer Program for Regulatory Compliance Part I |
 |
The first part focuses on the foundations of such a TTT program, addressing the processes involved in preparing for, conducting, and documenting the various sessions that make up the TTT program. Read More |
| |
|
|