| This Month On IVT Network |
| Change Control Case Study #1: Change Control Audit Finding |
 |
Citing an actual audit finding, Joe Zec discusses potential corrective and preventive actions for a change control citation. Read More |
| LATEST JVT JOURNAL ARTICLES |
| Validation Boot Camp Part II |
 |
In the second part of a presentation from Validation Week Canada, Dawn Tavalsky analyzes the basics of risk management; cleaning validation, including cleaning soils and equipment; the basics of the US Food and Drug Administration's approach to process validation; and the basics of quality systems.
Read More |
| Process Performance Qualification: Selecting the Optimal Sampling Plan |
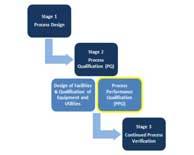 |
Process validation is defined as the collection and evaluation of data from the process design stage through the commercial production that establishes scientific evidence that a process is capable of consistently delivering quality product and thereby also assuring reliability of supply. Read More
|
| API Pharmaceutical Water Systems Part I: Water System Design |
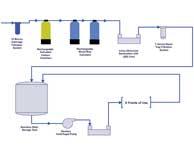 |
The design of a pharmaceutical water system for the active pharmaceutical ingredient (API) industry will depend on the type of API process. Read More |
| Integrating Risk Management into Computer System Validation |
 |
The last decade has brought about a number of changes to how pharmaceutical companies address validation. These changes have been brought about primarily by regulatory changes and the economy. Read More |
|
| LATEST GXP JOURNAL ARTICLES |
| Application of the GLP Regulations: Lessons from Warning Letters II: Responsibilities of Test Facilities and Contract Testing Laboratories |
 |
This paper will discuss an area of GLP that has generated a fair amount of conflicting discussions because of the desire on the part of study sponsors to avoid the cost of complete studies and on the part of contract testing laboratories that seek to accommodate client’s wishes. Read More
|
| QC Microbiology, GMP, and Social Media: Results of an Industry Survey |
 |
The use of social media has exploded in recent years. This has been a disruptive change, affecting the recording, broadcast, and print industries, among others. Read More |
| Transcending Compliance |
 |
Almost daily, we see new reports surfacing of failure to comply with current good pharmaceutical practices (cGxPs). Compliance remains an elusive goal for many participants in the global pharmaceutical business. Read More |
| Mitigating Risk Associated with Multitasking |
 |
Multitasking has taken on broad definitions, including doing more than one task at the same time, dealing with shifting priorities, managing multiple projects, doing more work than time permits, and many other situations. Read More |
| |
|
|