| This Month On IVT Network |
| Validation Week is the Global Leader in Validation Training! |
 |
Don't forget your special discount to attend the 19th Annual Validation Week, October 22-24, 2013 in San Diego. IVT Network Members Save $500 on registration using promo code IVTSUB
Find out why 300 of your colleagues attend each year. Read More |
| LATEST JVT JOURNAL ARTICLES |
| Sterile Barrier Systems: Managing Changes and Revalidations |
 |
The medical device package design and packaging process are critical steps for the production and distribution of terminally sterilized medical devices.
Read More |
| Use of the Eurachem (Quam) Guide on the Analytical Validation for Inorganic Anion Analysis by Capillary Ion Electrophoresis |
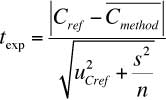 |
Capillary ion electrophoresis (CIE) with indirect ultraviolet (UV) detection was used for separation and quantification of several inorganic anions such as bromide, chloride, sulphate, nitrite, nitrate, fluoride, and phosphate in different natural and wastewater samples. Read More
|
| Quality-by-Design for Analytical Procedures: Introduction |
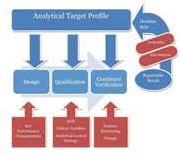 |
A new approach to analytical procedures has arrived. For production processes, quality-by-design (QbD) is being used successfully; the same QbD approach can be applied to analytical procedures. Read More |
| Integrating Risk Management into Computer System Validation |
 |
The last decade has brought about a number of changes to how pharmaceutical companies address validation. These changes have been brought about primarily by regulatory changes and the economy. Read More |
|
| LATEST GXP JOURNAL ARTICLES |
| Application of the GLP Regulations: Lessons from Warning Letters II: Responsibilities of Test Facilities and Contract Testing Laboratories |
 |
This paper will discuss an area of GLP that has generated a fair amount of conflicting discussions because of the desire on the part of study sponsors to avoid the cost of complete studies and on the part of contract testing laboratories that seek to accommodate client’s wishes. Read More
|
| QC Microbiology, GMP, and Social Media: Results of an Industry Survey |
 |
The use of social media has exploded in recent years. This has been a disruptive change, affecting the recording, broadcast, and print industries, among others. Read More |
| Transcending Compliance |
 |
Almost daily, we see new reports surfacing of failure to comply with current good pharmaceutical practices (cGxPs). Compliance remains an elusive goal for many participants in the global pharmaceutical business. Read More |
| Mitigating Risk Associated with Multitasking |
 |
Multitasking has taken on broad definitions, including doing more than one task at the same time, dealing with shifting priorities, managing multiple projects, doing more work than time permits, and many other situations. Read More |
| |
|
|