| This Month On IVT Network |
| Writing Validation Requests and Validation Plans |
 |
Paul Pluta, Ph.D., explains the process of connecting Stage 1 and Stage 2 of process validation through properly written and well formed validation requests and validation plans. Read More |
| LATEST JVT JOURNAL ARTICLES |
| Method Suitability Control Studies for Microbial Testing: Quantitative Comparisons |
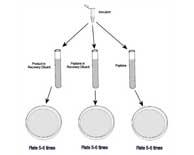 |
The measurement of microbial kill requires the ability to measure the number of surviving microorganisms with time after exposure to the antimicrobial agent.
Read More |
| Bacterial Adhesion: an Introduction |
 |
The phenomenon of bacterial adhesion is an important phenomenon for those working within the pharmaceutical and healthcare sectors to consider. Read More
|
| FDA Plans to Tighten Regulations Concerning Defibrillators |
 |
The U.S. Food and Drug Administration today [March 22, 2013] issued a proposed order aimed at helping manufacturers improve the quality and reliability of automated external defibrillators (AEDs). Read More |
| Degradation of Pharmaceutical Solids Accelerated by Changes in Both Relative Humidity and Temperature and Combined Storage Temperature and Storage Relative Humidity (T×h) Design Space for Solid Products |
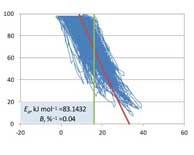 |
As noted in a previous report, achieving quality by design (QbD), rather than by testing to demonstrate compliance with specifications, is a concept in quality assurance that evolved in the last decade of the twentieth century.Read More |
|
| LATEST GXP JOURNAL ARTICLES |
| Analysis of Recent Drug GMP Inspections by FDA and AIFA: Different Approaches and Significant Trends |
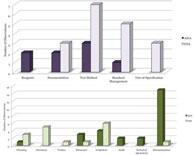 |
Companies regulated under good manufacturing practices (GMPs) are routinely inspected by health authorities in order to ensure the quality, safety, and efficacy of their products. Read More
|
| Implementing an Effective Provider Qualification Program at the Contract Laboratory |
 |
This paper is intended to provide useful hands-on guidance regarding the process of provider qualification. Read More |
| Ownership Responsibilities for the Pharmaceutical Quality Management System: Setting New Expectations |
 |
The pharmaceutical quality management system (QMS) is comprised of policies, standards, procedures, and other approved instructions that declare how a firm intends to apply company requirements, laws, and regulations to their operations. Read More |
| Mitigating Risk Associated with Multitasking |
 |
Multitasking has taken on broad definitions, including doing more than one task at the same time, dealing with shifting priorities, managing multiple projects, doing more work than time permits, and many other situations. Read More |
| |
|
|