| This Month On IVT Network |
| Complaint Handling – Consider the Patient’s Perspective |
 |
Mark Perkins leads the audience on an engaging and interactive session that considers a viewpoint of product complaints that is all to often missing from the life sciences industry – the patient's. Read More |
| LATEST JVT JOURNAL ARTICLES |
| Method Suitability Control Studies for Microbial Testing: Quantitative Comparisons |
 |
The measurement of microbial kill requires the ability to measure the number of surviving microorganisms with time after exposure to the antimicrobial agent.
Read More |
| Bacterial Adhesion: an Introduction |
 |
The phenomenon of bacterial adhesion is an important phenomenon for those working within the pharmaceutical and healthcare sectors to consider. Read More
|
| FDA Plans to Tighten Regulations Concerning Defibrillators |
 |
The U.S. Food and Drug Administration today [March 22, 2013] issued a proposed order aimed at helping manufacturers improve the quality and reliability of automated external defibrillators (AEDs). Read More |
| Degradation of Pharmaceutical Solids Accelerated by Changes in Both Relative Humidity and Temperature and Combined Storage Temperature and Storage Relative Humidity (T×h) Design Space for Solid Products |
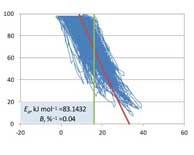 |
As noted in a previous report, achieving quality by design (QbD), rather than by testing to demonstrate compliance with specifications, is a concept in quality assurance that evolved in the last decade of the twentieth century.Read More |
|
| LATEST GXP JOURNAL ARTICLES |
| Integration of Risk Management Principles into the Quality System: Risk-based Impact Assessment |
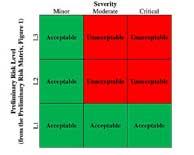 |
As the principles of quality risk management have become increasingly pervasive in the biopharmaceutical and medical device industries, a point of saturation has been reached with respect to the risk toolkit. Read More
|
| Application of the GLP Regulations: Lessons From Warning Letters I: General Introduction and Personnel Qualifications |
 |
The general structure of these letters will be discussed followed by a discussion of specific portions of the regulations. Read More |
| The Risk Mitigation Continuum and GMP Compliance |
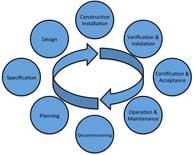 |
This paper presents the case that good manufacturing practice (GMP) compliance is basically the continuous mitigation of risk to the consistent quality of the drug product throughout the manufacturing lifecycle. Read More |
| Foreign Companies Beware–FDA is on a Regulatory "Warpath" |
 |
Over the past year or so, you can see that US Food and Drug Administration has been taking a very "proactive" inspectional approach. Read More |
| |
|
|