| This Month On IVT Network |
| The Product Recall Process |
 |
At the 8th Annual Product Recalls Summit East, David Bloch, J.D., discusses the legal definition of a recall, and how to successfully and quickly work through the product recall process. Read More |
| LATEST JVT JOURNAL ARTICLES |
| Biopharmaceutical Cleaning Validation: Acceptance Limits for Inactivated Product Based on Gelatin as a Reference Impurity |
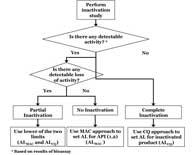 |
Biopharmaceutical cleaning and sterilization processes denature and degrade the active pharmaceutical ingredient (API) into fragments that are pharmacologically inactive.
Read More |
| cGMPS for Combination Products: 21 CFR Part 4—Final Rule |
 |
The US Food and Drug Administration released its Code of Federal Regulations Title 21 Part 4, Current Good Manufacturing Practice Requirements for Combination Products, on January 22, 2013.
Read More
|
| Establishing a Complete Set of Target, Alert, and Action Limits for Microbial Counts in Purified Water |
 |
Purified water (PW) is probably the only raw material essentially produced for internal use by pharmaceutical and other healthcare companies. Read More |
| Electronic Versus Paper Change Management System–Advantages and Disadvantages |
 |
Change is always around. This is especially true in the current globalized economic landscape wherein companies are facing changes on daily basis.
Read More |
|
| LATEST GXP JOURNAL ARTICLES |
| Cleaning Validation Residue Limits–How Clean is Clean? |
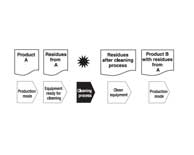 |
Cleaning residue limits for cleaning validation must be determined based on scientific and technical principles. Fourman and Mullen established the foundation for what has become the standard industry approach to setting limits for residual actives. Read More
|
| China's 2011 GMP Regulations–Combatting Poor API/Drug Quality |
 |
It is estimated that China manufactures about 80% of the active pharmaceutical ingredients (APIs) imported into the United States and the European Union (EU) for use in the manufacture of finished dosage forms. Read More |
| cGMP Equipment, Instruments, and Calibration |
 |
Equipment and instrumentation in facilities governed by current good manufacturing practices (cGMPs) must remain in a validated/qualified state. Read More |
| |
|
|